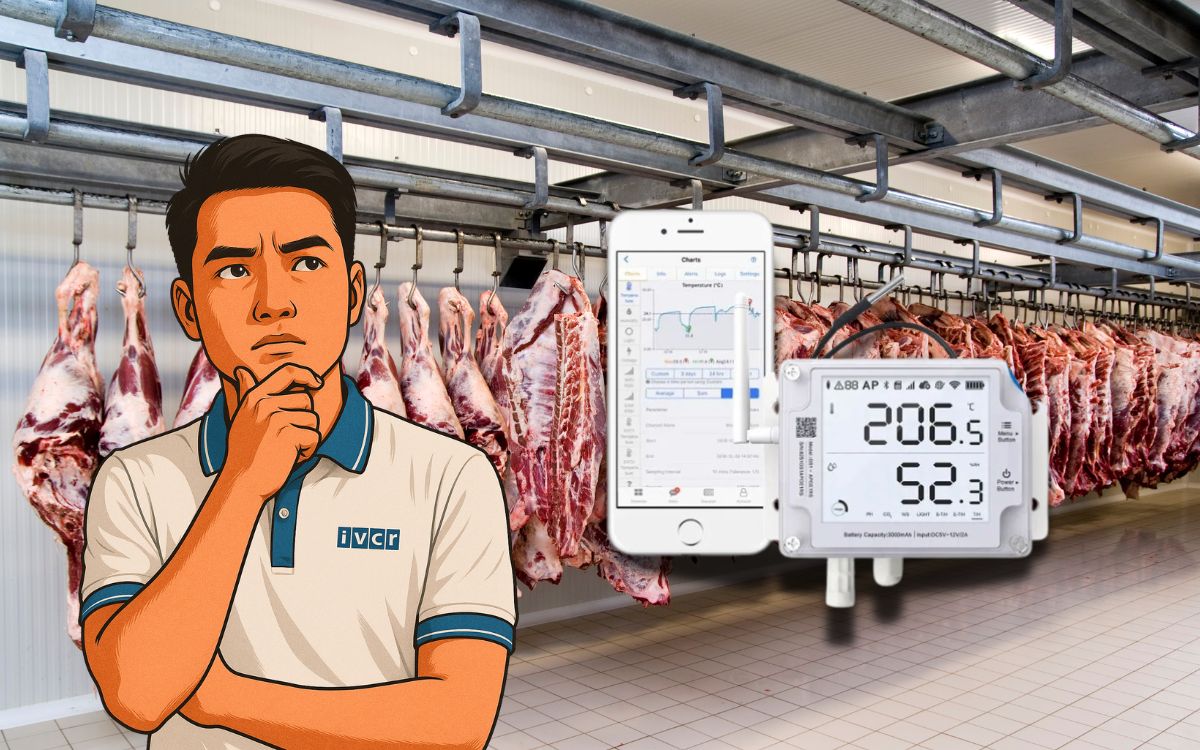
- 1. Why is it necessary to monitor temperature and humidity in cold storage?
- 2. Technical requirements for monitoring cold storage
- 3. What should a cold storage monitoring system include?
- 4. Temperature and Humidity Monitoring Solutions by Industry
- 5. Benefits of Using a Smart Temperature and Humidity Monitoring System
- 6. Frequently Asked Questions About Cold Storage Monitoring Systems
- 7. Need Advice on a Standard-Compliant Cold Storage Monitoring System?
1. Why is it necessary to monitor temperature and humidity in cold storage?
Cold storage is a critical link in the preservation chain for sensitive goods such as pharmaceuticals, food, or high-end cosmetics. If temperature and humidity are not precisely controlled, it can lead to quality degradation, reduced efficacy, or even render products unusable.
For pharmaceuticals: Even a 1°C deviation is unacceptable
Many types of vaccines, semi-finished raw materials, or injectable drugs require strict storage at 2-8°C, while others need deep-freeze conditions (-20°C, -80°C). Without continuous monitoring, even a 15-minute power outage or a slight temperature fluctuation can lead to a full batch being discarded, causing severe losses.
The GDP (Good Distribution Practices) standard requires pharmaceutical cold storage to be monitored 24/7 with early warning capabilities and data logging for audit purposes.

For food: Risk of food safety issues
Fresh meat, dairy, seafood, or produce are all highly sensitive to fluctuations in temperature and humidity. Bacteria can multiply quickly when the temperature exceeds the safe range, leading to foodborne illnesses or product spoilage without timely detection.
ISO 22000 and HACCP standards mandate temperature and humidity monitoring and data storage as part of the hazard control system.
Lack of monitoring = chain reaction of risk
Temperature and humidity deviations in cold storage can lead to:
- Failed qualification or validation
- Shipment rejection during export inspections
- Loss of credibility with partners or regulatory bodies
Monitoring temperature and humidity is not just a recommended best practice—it is a mandatory requirement in high-risk industries.
2. Technical requirements for monitoring cold storage
A compliant cold storage monitoring system is not just about “having sensors” or “recording temperature.” It must meet strict technical criteria based on pharmaceutical, food, or cold-chain logistics requirements.
High accuracy - the lower the margin of error, the better
For sensitive goods like vaccines or fresh meat, even a ±1°C deviation can compromise quality. Therefore, sensors must ensure:
- Temperature accuracy: ±0.2°C or ±0.5°C depending on the industry
- Humidity accuracy: ±2%RH or ±3%RH for high-sensitivity environments
In the pharmaceutical industry, sensors typically require calibration certificates according to ISO 17025.

Appropriate measurement range for each type of cold storage
|
Storage type |
Temperature range |
Humidity range |
|
Food cold storage |
-18°C to +5°C |
30%-80% RH |
|
Pharmaceutical storage |
2°C to 8°C (stable) |
40%-70% RH |
|
Deep-freeze (e.g., COVID vaccines) |
-70°C to -20°C |
Not humidity-dependent |
Choosing the wrong sensor range may result in incorrect data or failed alerts.
Continuous data logging & storage
A good system should:
- Log data every 1-5 minutes (depending on needs)
- Store data for at least 3-6 months (locally or on the cloud)
- Export reports in PDF/Excel formats for audits (WHO, GMP compliant)
Continuous logging helps businesses verify records in case of disputes or sudden inspections by authorities.
Automated alerts - not just passive recording
Monitoring isn’t just about “recording”; it must alert when incidents occur, including:
- Threshold breaches in temperature or humidity
- Power outages or internet disconnections
- Notifications sent via email, SMS, or mobile app
A good system must respond quickly to help technicians address issues before products are affected.
3. What should a cold storage monitoring system include?
An effective cold storage temperature and humidity monitoring system is not a standalone device but a set of synchronized components, from sensors and data acquisition devices to a centralized monitoring platform.
|
Device |
Primary function |
|
Temperature & humidity sensor |
Placed directly in the storage or among pallets to measure actual conditions |
|
Data logger |
Records data continuously (1-5 min intervals) and stores it internally if disconnected |
|
Data gateway |
Collects data from sensors/loggers and transmits it via Wi-Fi, LAN, or 4G |
|
Monitoring software |
Real-time dashboard, trend graphs, scheduled reports, and alert notifications |
Implementation notes:
- Number of sensors: Place at least 3 per area (front - center - back) for balanced evaluation
- System scalability: Should allow adding more sensors as the warehouse expands or is sectioned
- Device compatibility: Prefer open systems that integrate with BMS or warehouse management software
For cold storage used in pharmaceutical or food supply chains, having complete reporting, electronic data retention, and calibration certificates is essential to pass compliance inspections.
4. Temperature and Humidity Monitoring Solutions by Industry
Depending on the nature of the products and quality control regulations, each industry has specific requirements for the design and implementation of a cold storage monitoring system. Below are optimal solutions for each sector:

Pharmaceutical Industry: GDP-compliant - zero tolerance for errors
In the pharmaceutical industry, especially for storing vaccines, biologicals, and sensitive raw materials, the monitoring system must ensure:
- 24/7 continuous data recording and storage
- Immediate alerts when temperature exceeds the 2-8°C range
- Automatic PDF/Excel reports for each batch upon dispatch
- Data retention for at least 5 years to support validation
- Sensors must be calibrated periodically according to ISO 17025
This is a mandatory requirement under WHO and EU GDP (Good Distribution Practice) standards.
Food Industry: Stable temperature control - avoid spoilage risks
For fresh, frozen, or perishable food, the monitoring system should:
- Measure temperature continuously in high-risk areas (warehouse doors, near cooling fans, corners)
- Provide alerts before temperature limits are exceeded
- Integrate with the warehouse management system (WMS) to stop shipment if temperature is out of range
- Send alerts to operations and logistics teams via App/SMS
In HACCP and ISO 22000 systems, monitoring data is crucial evidence to confirm control of critical control points (CCP).
Cosmetics Industry: Focus on humidity and flexibility
Some product lines like serums, creams, and perfumes have volatile components that may degrade if humidity exceeds thresholds. Optimal solutions include:
- Use wireless temperature and humidity sensors for flexible placement without affecting the layout or aesthetics of the warehouse
- Continuously monitor humidity in the 40%-70% RH range depending on the product
- Integrate soft alerts (via Email/App) for production and maintenance management
- Use visual monitoring software for easy oversight by QA/QC
The system should support scheduled reporting by zone to assist with internal inspections and ISO 22716 audits.
5. Benefits of Using a Smart Temperature and Humidity Monitoring System
Implementing a smart monitoring system for cold storage not only helps meet compliance requirements but also brings significant value in operations, finances, and quality management for businesses. Key benefits include:
Early incident detection - minimize damage
- Immediate alerts when abnormal temperature changes are detected, allowing technicians to act before goods are affected
- Rapid detection of power outages, cooling system failures, or prolonged door openings
- Minimize the risk of losing an entire batch - especially critical in the pharmaceutical and export food industries
Support for traceability - transparency in audits and inspections
- Easily export data reports by batch, date, or storage zone
- Facilitate preparation for GMP, ISO, HACCP, FDA inspections
- Event logs help analyze root causes of incidents or complaints
In pharmaceutical plants, monitoring traceability is a mandatory requirement in system validation (Validation - URS, DQ, IQ, OQ, PQ)

Optimize operational costs and staffing
- No need for manual temperature checks → reduced labor costs
- Lower energy consumption by identifying inefficient cooling system performance
- Avoid losses caused by uneven temperature distribution in the warehouse
Centralized management - easy remote monitoring
- Access the system anytime, anywhere via mobile app or web browser
- The system can scale across multiple warehouses or branches - ideal for enterprises with distributed operations
- Integrates with ERP or warehouse management software for data synchronization
6. Frequently Asked Questions About Cold Storage Monitoring Systems
Is it mandatory to install a monitoring system for cold storage?
Yes. If your warehouse stores pharmaceuticals, vaccines, or export food products, it is mandatory to have a temperature and humidity monitoring system. This is required under standards such as:
- GMP/GDP for pharmaceuticals
- ISO 22000 and HACCP for food products
Additionally, monitoring data serves as crucial evidence during audits, routine inspections, or product complaint investigations.
Does the monitoring system work during power outages?
Yes, depending on the configuration. To ensure continuous operation during power outages:
- Choose sensors with backup batteries or standalone data loggers
- Gateways should have UPS (Uninterruptible Power Supply) if connected via LAN
- Check the actual system backup time to ensure no data is lost
Can cold storage data be viewed on a smartphone?
Absolutely, if the system includes remote monitoring software. Modern solutions typically support:
- Mobile apps (Android/iOS) for real-time tracking
- Cloud/Web app access
- Customizable alerts via SMS/Email/Push Notifications
This feature is especially useful for operations, QA/QC teams, or remote managers overseeing multiple warehouses.
How often should sensors be calibrated?
Calibration frequency depends on the industry and applicable standards:
- For pharmaceuticals: every 6 months according to ISO 17025 (or as specified in URS)
- For food or standard warehouses: every 6-12 months depending on usage and risk level
Once calibration is completed, records and calibration labels should be retained for inspection purposes.
7. Need Advice on a Standard-Compliant Cold Storage Monitoring System?
Are you managing a warehouse for pharmaceuticals, frozen food, or cosmetics that require humidity control?
Don't wait until product loss to think about monitoring. A smart monitoring system helps you pass audits, protect product quality, and enhance your credibility with global partners.
Contact VCR’s technical team for consultation:
- Free consultation tailored to your industry
- Support for URS development - device calibration according to ISO 17025
- Integrated remote monitoring software - automated reporting
Hotline: 090.123.9008
Email: [email protected]
Website: https://phongsachthucpham.com/
Diep VCR
Vietnam Cleanroom (VCR) là một doanh nghiệp hàng đầu tại Việt Nam chuyên cung cấp thiết bị và giải pháp phòng sạch. Với hơn 10 năm kinh nghiệm phục vụ các dự án phòng sạch đạt tiêu chuẩn GMP, VCR tự hào mang đến các thiết bị kỹ thuật cao như: đồng hồ chênh áp, khóa liên động, đèn phòng sạch, Pass Box, FFU (Fan Filter Unit), buồng cân, HEPA Box, Air Shower, cửa thép phòng sạch, tủ cách ly (ISOLATOR), và nhiều loại phụ kiện chuyên dụng khác
Không chỉ là nhà cung cấp thiết bị, VCR còn là đơn vị phân phối độc quyền các sản phẩm từ các thương hiệu quốc tế như LENGE và BLOCK Technical, đồng thời cung cấp các giải pháp phòng sạch toàn diện cho các lĩnh vực như dược phẩm, điện tử, y tế, thực phẩm và mỹ phẩm. VCR có đội ngũ chuyên gia giàu kinh nghiệm, kiến thức chuyên sâu về phòng sạch, hỗ trợ tư vấn về tiêu chuẩn, thiết kế, thi công và vận hành phòng sạch theo chuẩn ISO, GMP, HACCP, ISO 14644
VCR hướng đến trở thành thương hiệu quốc dân trong ngành phòng sạch, với mạng lưới cung ứng rộng khắp, VCR có các văn phòng tại Hà Nội, TP. HCM, đáp ứng mọi yêu cầu từ xây dựng đến nâng cấp môi trường sản xuất đạt chuẩn
Email: [email protected]
Điện thoại: (+84) 901239008
Địa chỉ:
VP Hà Nội: 9/675 Lạc Long Quân, P. Xuân La, Q. Tây Hồ, TP. Hà Nội
VP Hồ Chí Minh: 15/42 Phan Huy Ích, P.15, Q. Tân Bình, TP.HCM
Hãy liên hệ với VCR để tìm hiểu thêm về lĩnh vực phòng sạch hiệu quả nhất nhé!